- This topic is empty.
-
AuthorPosts
-
10/07/2025 at 17:43 #7429
In the realm of industrial by-products, phosphogypsum stands out as a significant material due to its substantial production volume and the challenges it poses for sustainable utilization. In this blog post, Metash, a high performance UV Visible spectrophotometer manufacturer, will share the application of double beam UV-visible spectrophotometer for phosphorus content in phosphogypsum detection.
Introduction to Phosphogypsum
Phosphogypsum is a by-product generated during the production of wet-process phosphoric acid. Its primary component is calcium sulfate dihydrate (CaSO₄·2H₂O), but it also contains trace amounts of phosphorus, fluorine, organic substances, and radioactive materials. The presence of phosphorus in phosphogypsum is particularly noteworthy as it significantly impacts its potential applications. China, being a major producer of phosphate fertilizers, generates over 70 million tons of phosphogypsum annually, with a cumulative stockpile reaching 820 million tons. The comprehensive utilization rate of phosphogypsum is only about 50%, making it a pressing environmental issue in the phosphorus chemical industry.

Importance of Phosphorus Content Detection
The phosphorus in phosphogypsum exists in three forms: soluble phosphorus, insoluble phosphorus, and phosphorus in crystal lattice. The accurate determination of phosphorus content, especially the total and water-soluble forms of phosphorus pentoxide (P₂O₅), is crucial for assessing the usability of phosphogypsum. This information helps in devising appropriate pre-treatment methods such as washing, calcination, and lime neutralization to mitigate the adverse effects of impurities.
Experimental Methodology
Instruments and Reagents
The experiment employs a Q-6 double beam UV-visible spectrophotometer, a heating plate, an analytical balance (with a precision of 0.0001 g), and standard reagents including nitric acid, hydrochloric acid, potassium dihydrogen phosphate, ammonium vanadate, and ammonium molybdate tetrahydrate.

Determination of Water Content
To determine the water content in phosphogypsum, a sample of 10.0000 g is weighed and placed in a pre-weighed weighing bottle. This sample is then dried at 40°C for 2 hours in a constant temperature drying oven. After cooling in a desiccator, the sample is reweighed. This process is repeated until the weight remains constant, indicating that all the water has been removed. The water content is calculated using the formula:
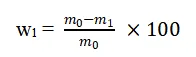
where w1 is the water content (%), m0 is the initial weight of the phosphogypsum (g), and m1 is the weight after drying (g).
Color Reagent Preparation
A color reagent is prepared by dissolving 0.78 g of ammonium vanadate in 64 mL of nitric acid solution (1:1 v/v) and diluting to 100 mL with water. Separately, 10.26 g of ammonium molybdate is dissolved in water and diluted to 100 mL. These two solutions are then mixed in equal volumes.
Sample Pre-treatment
For the determination of total P₂O₅, 3.0000 g of phosphogypsum is weighed and placed in a 250 mL beaker. The sample is moistened with a small amount of ultra-pure water, followed by the addition of 30 mL of hydrochloric acid and 10 mL of nitric acid. The mixture is heated to boiling and maintained at a gentle boil for 15 minutes. After cooling, the solution is transferred to a 250 mL volumetric flask, diluted to the mark, and filtered. A 25 mL aliquot of the filtered solution is taken and diluted to 100 mL with the addition of 10 mL of nitric acid solution (1:1 v/v) and 20 mL of ultra-pure water. Subsequently, 15 mL of the color reagent is added, and the solution is diluted to 100 mL and left to stand for 30 minutes before measurement.
For the determination of water-soluble P₂O₅, 5.0000 g of phosphogypsum is weighed and placed in a mortar. The sample is ground for 15 minutes with 50 mL of ultra-pure water. The ground sample is then transferred to a 250 mL volumetric flask, diluted to the mark, and left to stand for 30 minutes. The solution is filtered, and a 25 mL aliquot is taken and diluted to 100 mL with the addition of 10 mL of nitric acid solution (1:1 v/v) and 20 mL of ultra-pure water. Finally, 15 mL of the color reagent is added, and the solution is diluted to 100 mL and left to stand for 30 minutes before measurement.
Standard Curve Construction
A standard solution of P₂O₅ is prepared by dissolving 0.1917 g of dried potassium dihydrogen phosphate (dried at 110°C for 2 hours) in a 100 mL volumetric flask. The solution is diluted to the mark with ultra-pure water and contains 1000 mg/L of P₂O₅. Aliquots of this standard solution (0.0 mL, 1.0 mL, 2.0 mL, 3.0 mL, 5.0 mL, 10.0 mL) are transferred to 100 mL volumetric flasks, and each is diluted to the mark with the addition of 10 mL of nitric acid solution (1:1 v/v) and 40 mL of ultra-pure water. Subsequently, 15 mL of the color reagent is added to each flask, and the solutions are diluted to 100 mL and left to stand for 30 minutes. The absorbance of these solutions is measured at 480 nm and 500 nm using the UV-visible spectrophotometer. The difference in absorbance values is plotted against the concentration to construct a standard curve.
Sample Testing
The absorbance of the prepared sample solutions is measured at 480 nm and 500 nm using the UV-visible spectrophotometer. The difference in absorbance values is used to determine the concentration of P₂O₅ in the samples. The total and water-soluble P₂O₅ contents are calculated using the following formulas:
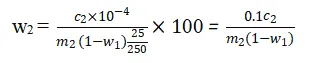
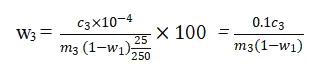
where w2 and w3 are the total and water-soluble P₂O₅ contents (%), c2 and c3 are the concentrations of P₂O₅ in the samples (mg/L), m2 and m3 are the masses of the phosphogypsum samples (g), V is the volume of the sample solution (L), and w1 is the water content (%).
Results and Discussion
The standard curve constructed from the P₂O₅ standard solutions exhibits a linear relationship with a correlation coefficient R2>0.999. The total and water-soluble P₂O₅ contents in the phosphogypsum samples were determined to be 0.40% and 0.21%, respectively. The precision and accuracy of the measurements were found to be satisfactory, indicating the reliability of the UV-visible spectrophotometric method.
Conclusion
The use of the double beam UV-visible spectrophotometer for detecting the phosphorus content in phosphogypsum offers a precise and reliable method for assessing its usability. This technique, in accordance with the standard JC/T 2073-2011, provides valuable insights into the sustainable utilization of phosphogypsum. By accurately determining the phosphorus content, appropriate pre-treatment methods can be applied to enhance the usability of this industrial by-product, thereby contributing to the sustainable development of the phosphorus chemical industry.
http://www.metashcorp.com
Metash -
AuthorPosts
- You must be logged in to reply to this topic.

 Google hit with record EU fine over Shopping service
Google hit with record EU fine over Shopping service  Business booming for giant cargo planes
Business booming for giant cargo planes  Trump-Putin: The understandable story
Trump-Putin: The understandable story